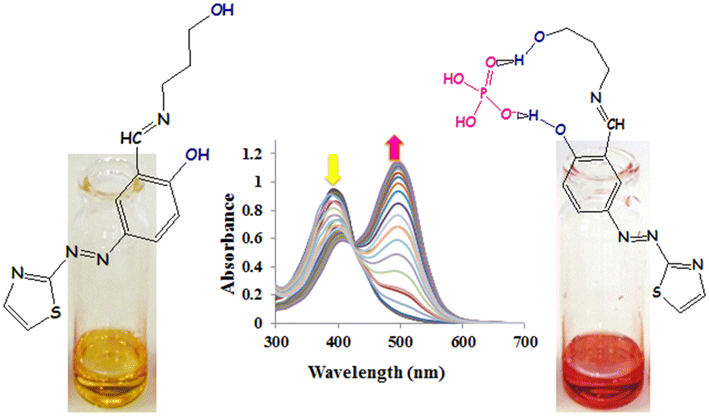
A novel receptor for detection of Zn2+ metal ion and F−, H2PO4 − and AcO− anions in aqueous media: a DFT studyMasoumeh Orojloo, Raziyeh Arabahmadi, Fatemeh Naderi, Fatemeh Parchegani, Mohammad Solimannejad, Peyman Zolgharnein, and Saeid Amani Arak University, Arak, Iran
E-mail: s-amani@araku.ac.ir Abstract: A new colorimetric chemosensor L, containing electron-donating moieties attached to the thiazole-based Schiff base was synthesized. The optical and colorimetric sensing properties of L for anions and cations were investigated using naked eye, UV–Vis and computational studies. The receptor L displays visual changes towards anions like F−, H2PO4 − and AcO− and also towards cation such as Zn2+ in DMSO. Other anions such as Cl −, Br−, I−, NO3 − and HSO4 − did not cause any color change. On the addition of other metal ions such as Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Cd2+, Hg2+ and Pb2+, the receptor did not show any significant change. The binding constant (K a ) and stoichiometry of the host–guest complex formed were calculated by the Benesi–Hildebrand (B–H) plot and Job’s plot method, respectively. Computational studies and UV–Vis titration were further used to emphasize the sensing behavior of the receptor. Quantum chemical calculations and molecular studies using Density Functional Theory and Molecular Electrostatic Potential surface studies were carried out to supplement the experimental results and gain deeper insights about the structural as well as the spectral aspects of the complex. Receptor L proved to be a fluorescence and colorimetric Zn (II), fluoride (F−1), di-hydrogen phosphate (H2PO 4 −1 ) and acetate (AcO−1) sensor. This new chromogenic receptor shows a highly selective coloration for the above ions. The chemosensor showed a color change upon addition of Zn (II), or fluoride or di-hydrogen phosphate or acetate ions.
Keywords: Naked-eye detection ; Chemosensor ; DFT Calculations ; Thiazole Schiff base Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-017-0312-7
Chemical Papers 72 (3) 719–729 (2018) |
Friday, April 04, 2025 |
|||
© 2025 Chemical Papers |
||||