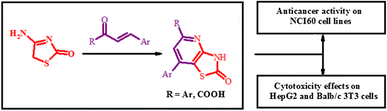
Synthesis and cytotoxicity of new thiazolo[4,5-b]pyridine-2(3H)-one derivatives based on α,β-unsaturated ketones and α-ketoacidsAndrii Lozynskyi, Borys Zimenkovsky, Lidia Radko, Sylwia Stypula-Trebas, Olexandra Roman, Andrzej K. Gzella, and Roman Lesyk Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
E-mail: dr_r_lesyk@org.lviv.net Abstract: A series of thiazolo[4,5-b]pyridin-2(3H)-one derivatives were obtained via [3 + 3]-cyclization of 4-amino-5H-thiazol-2-one and α,β-unsaturated ketones or α-ketoacids. The structures of newly synthesized compounds were established by spectral data and a single-crystal X-ray diffraction analysis. Target compounds were screened for their anticancer activity according to US NCI protocols and moderate inhibitory activity against the tested cell line was confirmed. 5-Phenyl-7-(pyridin-3-yl)-3H-thiazolo[4,5-b]pyridin-2-one (3) and 2-oxo-7-thiophen-2-yl-2,3-dihydrothiazolo[4,5-b]pyridine-5-carboxylic acid (12) were screened for their cytotoxicity effects on HepG2 and Balb/c 3T3 cells which revealed promising results using MTT, NRU and TPC assays.
Keywords: 4-Amino-5H-thiazol-2-one ; Thiazolo[4,5-b]pyridin-2(3H)-one ; X-ray study ; Cell lines ; HepG2 ; Balb/c 3T3 Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-017-0318-1
Chemical Papers 72 (3) 669–681 (2018) |
Sunday, November 24, 2024 |
|||
© 2024 Chemical Papers |
||||