Synthesis, spectroscopic characterization, X-ray structure, DFT calculations, and antimicrobial studies of diorganotin (IV) complexes of monotopic oxygen nitrogen donor Schiff baseShaukat Shujah, Saqib Ali, Nasir Khalid, Mohammad Jane Alam, Shabbir Ahmad, and Auke Meetsma Kohat University of Science and Technology, Kohat, Pakistan
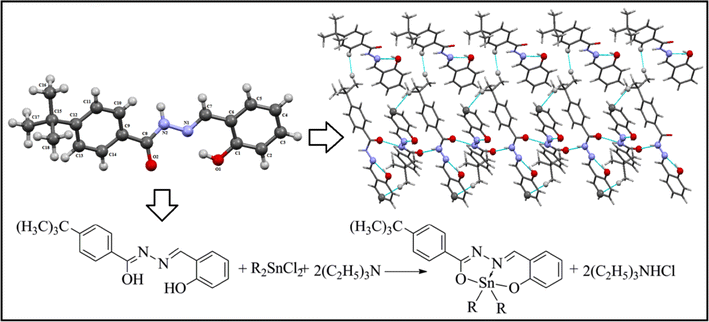
E-mail: shaukat.shujah@yahoo.com Abstract: Seven new diorganotin(IV) complexes, [Me2SnL] (1), [Et2SnL] (2), [(n-Bu2SnL] (3), [Ph2SnL] (4), [(n-Oct2SnL] (5), [tert-Bu2SnL] (6), and [n-BuClSnL] (7) have been synthesized from the reaction of N′-(2-hydroybenzylidene)-4-tert-butylbenzohydrazide (H2L) with the corresponding diorganotin(IV) dichloride/oxide or organotin(IV) chloride dihydroxide. The synthesized compounds were structurally characterized by FT-IR, multinuclear NMR (1H and 13C) spectroscopies, elemental analysis, mass spectrometry, DFT/(B3LYP) calculations, and, for ligand single crystal, X-ray diffraction analysis. Spectroscopic evidence affirms coordination of ligand to the dialkyltin(IV) moieties through oxygen nitrogen donor sites in iminol form. The 1 J(119Sn, 13C) coupling constants, 584-655 Hz and 2 J(119Sn-1H) coupling constant, 79 Hz for complex (1), suggest pentacoordination around Sn atom in solution. Single-crystal X-ray structure of ligand show its existence in amido form. Supramolecular architecture mediated by N(2)-H···O(2) and C-H…π interactions is formed in solid state. The DFT calculations have been performed to obtain various structure-based molecular properties as well as to support experimental results. The synthesized compounds were screened in vitro against various human pathogenic microbial strains. Escherichia coli and Staphylococcus aureus were most visibly inhibited by complexes (4) and (7). Highest antifungal activity was shown by compound (6) against Fusarium solani. Compound (3) displayed highest cytotoxicity among synthesized compounds with LD50 0.44μg/mL.
Keywords: Diorganotin(IV) complexes ; DFT calculations ; Antibacterial activity ; Antifungal ; Activity ; Cytotoxicity Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-017-0333-2
Chemical Papers 72 (4) 903–919 (2018) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||