Two cadmium(II) complexes derived from bidentate bis(benzoimidazol-2-ylmethyl)cyclohexane ligands: synthesis, crystal structures, spectroscopic and DFT calculationsRong-Kai Pan, Yun Wang, Jiang-Li Song, and Sheng-Gui Liu Lingnan Normal University, Zhanjiang, People’s Republic of China
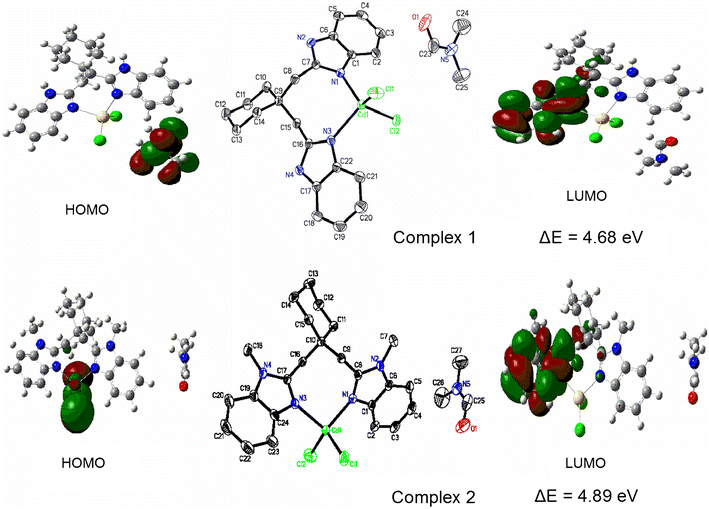
E-mail: wangyun203@hotmail.com Abstract: Two mononuclear cadmium(II) complexes [Cd(bbc)Cl2]·DMF(1) [bbc = 1,1-bis(1H-benzoimidazol-2-ylmethyl)cyclohexane] and [Cd(bmbc)Cl2]·DMF(2) [bmbc = 1,1-bis(1-methyl-benzoimidazol-2-ylmethyl)cyclohexane] were synthesized and characterized by physicochemical methods and X-ray single-crystal structure analysis. The central Cd(II) atoms of two complexes have distorted tetrahedral configurations with N2Cl2 binding sets. The 1D chain structures of complexes 1 and 2 are generated by π…π stacking interactions and C–H…π interactions, respectively. The fluorescence properties of both complexes have been investigated both in solution and solid state, and compared with the free ligands. Density functional theory (DFT) calculations at the B3LYP/3-21G and B3LYP/6-31G* levels of theory have been, respectively, carried out to investigate the optimized geometries of the complexes. Moreover, energy of HOMO and LUMO and natural bond orbital (NBO) calculations were evaluated and discussed. The calculated results show that both complexes reveal the covalent interactions between the coordinated atoms and Cd2+ ions. Two cadmium(II) complexes [Cd(bbc)Cl2]·DMF(1) and [Cd(bmbc)Cl2]·DMF(2) were synthesized and characterized by X-ray single-crystal structure analysis. The central Cd(II) atom of each complex has a distorted tetrahedral configuration with N2Cl2 binding sets, forming a 1D chain structure. The optimized geometry of both complexes have been obtained by DFT method at B3LYP/3-21G and B3LYP/6-31G* levels. Good agreement was found between the computational and the experimental results with B3LYP/3-21G//LANL2DZ basis sets. Keywords: Benzimidazole ; Cadmium(II) complex ; Crystal structure ; DFT calculations Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0475-x
Chemical Papers 72 (9) 2181–2191 (2018) |
Sunday, November 24, 2024 |
|||
© 2024 Chemical Papers |
||||