Two organically templated metal sulfate/selenate: IR spectroscopy, crystal structure, and thermal behaviourMarwa Abid Derbel, Ines Kadri, Houcine Naïli, and Walid Rekik Université de Sfax, Sfax, Tunisia
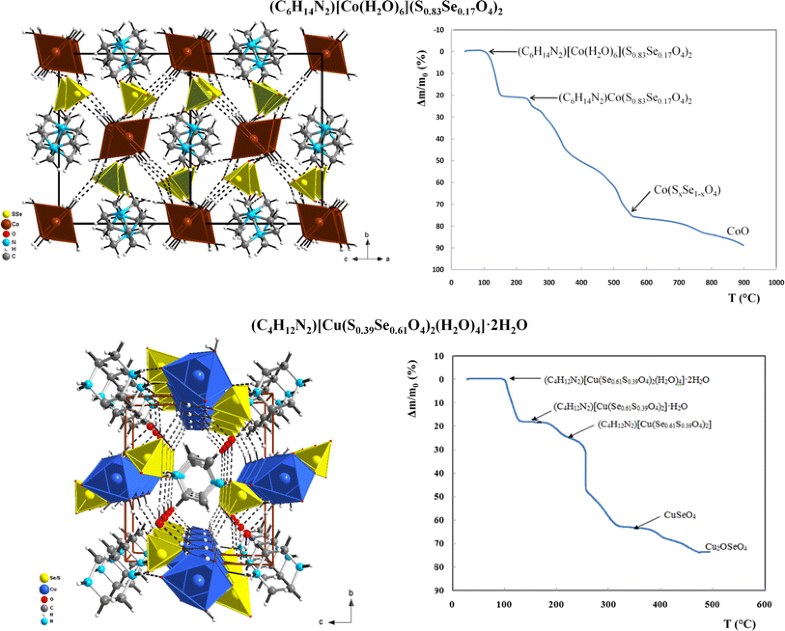
E-mail: walid.rekik@fss.usf.tn Abstract: Two organically templated transition metal sulfate/selenate compounds with following formula (C6H14N2)[Co(H2O)6](S0.83Se0.17O4)2 (1) and (C4H12N2)[Cu(H2O)4(S0.39Se0.61O4)2]·2H2O (2) have been synthesized and crystallographically characterized. Both coordination compounds adopt the monoclinic symmetry, P21/c space group with the following unit-cell parameters: a = 12.0669(13) Å; b = 12.1768(11) Å; c = 12.1158(12) Å; β = 104.023(4)° and Z = 4 for (1) and P21/n space group with the following unit-cell parameters: a = 6.9783(2) Å; b = 11.7101(4) Å; c = 10.6238(3) Å; β = 104.431(2)° and Z = 2 for (2). While compound (1) is fully supramolecular with a crystal structure consisting of metallic cations octahedrally coordinated by six water molecules [Co(H2O)6]2+, dabcodiium and sulfate/selenate (S0.39Se0.61O4)2− anions which are linked via Ow–H…O and N–H…O Hydrogen bonds, compound (2) exhibits a beginning of condensation and its atomic arrangement is built from anionic entity [Cu(H2O)4(S0.39Se0.61O4)2]2− and piperazinediium cations (C4H12N2)2+ linked together by hydrogen bonds only. Through this work, we can conclude that the partial substitution of sulfur by selenium does not affect the atomic arrangement and that the type of structure adopted by the new solid solution is that of the pure sulfate or selenate-based compound according to the rate of substitution. The thermal decomposition of both precursors proceeds through several stages giving rise to the cobalt oxide and to the copper oxyselenate as final product for (1) and (2), respectively. Keywords: Partial substitution sulfate/selenate ; Crystal structure ; Thermal decomposition ; Supramolecular chemistry ; Coordination compounds Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0503-x
Chemical Papers 72 (10) 2503–2512 (2018) |
Sunday, November 24, 2024 |
|||
© 2024 Chemical Papers |
||||