Optimization of process parameters using response surface methodology for Pd(II) extraction with quaternary ammonium salt from chloride medium: kinetic and thermodynamics studyVanee Mohdee, Kreangkrai Maneeintr, Thanaporn Wannachod, Suphot Phatanasri, and Ura Pancharoen Chulalongkorn University, Bangkok, Thailand
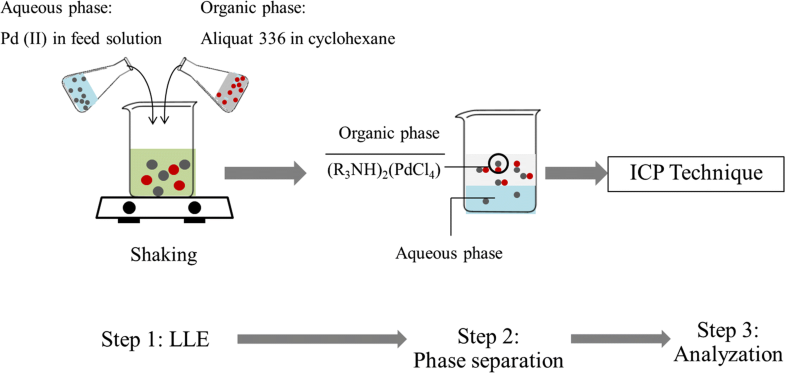
E-mail: s_phatanasri@yahoo.com Abstract: In this study, liquid–liquid extraction with Aliquat 336 in the presence of cyclohexane as a mobile carrier was investigated to purify Pd(II) from industrial wastewater. Kinetic and thermodynamics analysis showed that Pd(II) extraction was of first-order reaction: the reaction was endothermic reaction which was governed by the diffusion region. The influence of temperature on isotherm model was also investigated. With an increase in reaction temperature, all three isotherms Langmuir, Freundlich and Temkin isotherm become inaccurate. Results show that the Langmuir isotherm model was preferred for the study of the Pd(II) ion experimental isotherm. Response surface methodology was employed to study the five independent variables which have an effect on the percentage of extraction of palladium(II) ions as a dependent variable. Optimum extraction conditions for the five independent variables were as follows: Aliquat 336 concentration (0.6 M), pH of feed solution (2.0), stirring speed (600 rpm), reaction time (10 min.) and reaction temperature (318 K). Keywords: Optimization ; CCD ; RSM ; Pd(II) purification ; LLE ; Isotherm model Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0542-3
Chemical Papers 72 (12) 3129–3139 (2018) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||