An improved corrosion resistance of steel in hydrochloric acid solution using Hibiscus sabdariffa leaf extractNguyen To Hoai, Pham Van Hien, Nguyen Si Hoai Vu, Do Lam Son, Tran Van Man, Mai Dinh Tri, and Nguyen Dang Nam PetroVietnam University, Ba Ria City, Vietnam
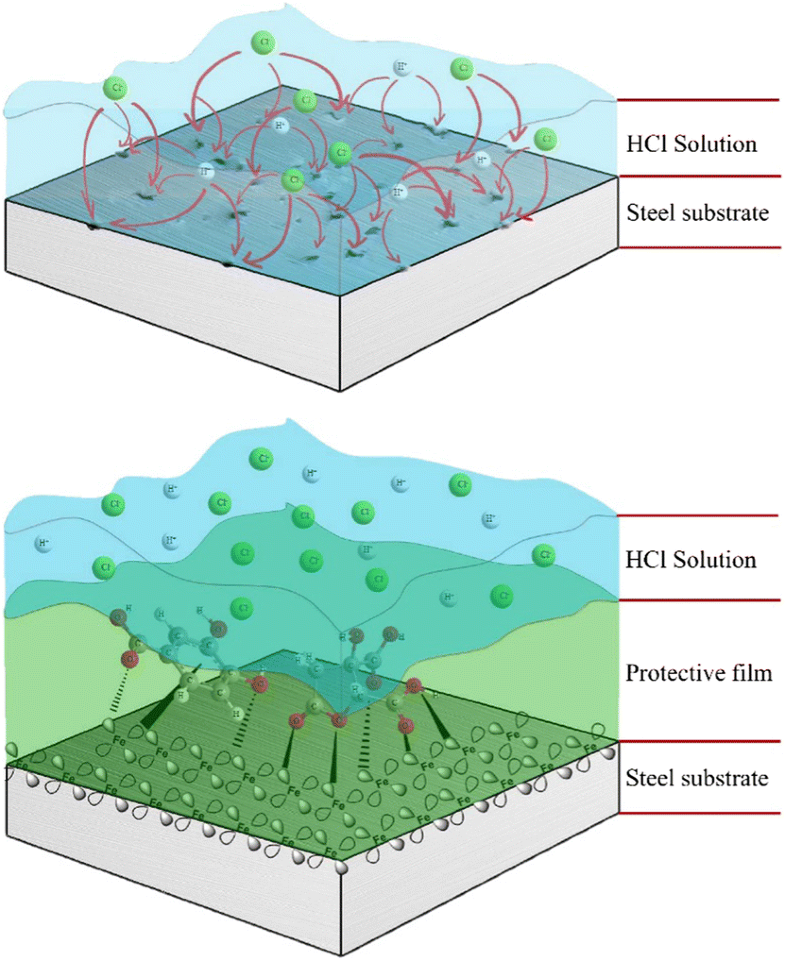
E-mail: nguyendangnam@dtu.edu.vn Abstract: The study used some sophisticated electrochemical techniques and surface analysis for corrosion research to unravel the inhibitive action of Hibiscus sabdariffa leaf extract on the corrosion of mild steel in 0.1 M HCl solution. The results indicate that the Hibiscus sabdariffa leaf extract acts as a mixed-type inhibitor and forms a defensive layer on the steel surface to enhance the corrosion resistance, which accounts for the diminished corrosion rate and reinforced polarization resistance. The highest inhibition efficiency of Hibiscus sabdariffa leaf extract was documented at 1500 ppm using electrochemical tests. Surface analysis indicated that a severe corrosion was observed on the steel surface exposed to solution without inhibitor addition, but less corrosion was observed on the steel surfaces exposed to the inhibited systems. Furthermore, an adsorbed layer and its components on the steel surfaces were also identified evidently by employing attenuated total reflectance Fourier transform infrared spectroscopy, scanning electron microscopy, and X-ray photoelectron spectroscopy. Molecular dynamic simulation was investigated to further understand the corrosion inhibition ability of Hibiscus sabdariffa leaf extract. An agreement of the electrochemical behaviors, surface observations, and molecular dynamic simulations demonstrated the good inhibition performance of Hibiscus sabdariffa leaf extract due to the formation of protective layer via the adsorption activities. Keywords: Mild steel ; Hibiscus sabdariffa ; Corrosion inhibitor ; Electrochemical techniques ; Surface analysis Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0649-6
Chemical Papers 73 (4) 909–925 (2019) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||