Electrode behavior of some triazole derivatives using convolutive voltammetry, chronoamperometry and differential pulse polarography techniquesAhmed A. Al Owais and Ibrahim S. El-Hallag King Saud University, Riyadh, Saudi Arabia
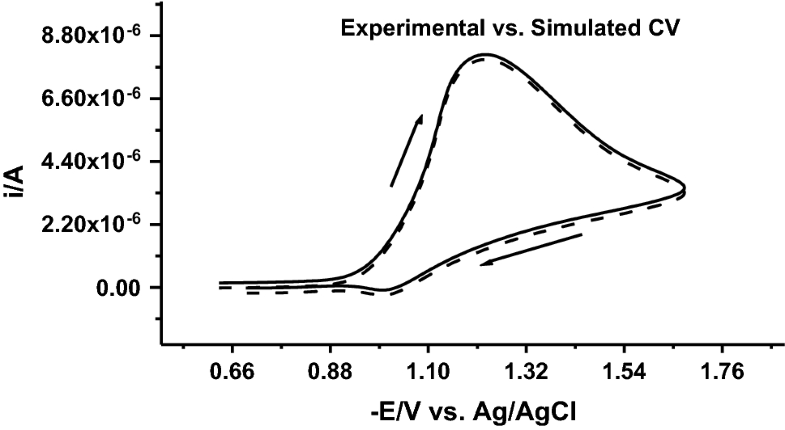
E-mail: i.elhallag@yahoo.com Abstract: Herein this work, summarize the behavior of the electrode reaction of some arylazomethine-1,2,3-triazole derivatives via convolutive sweep voltammetry, chronoamperometry and differential pulse polarography techniques in Britton Robinson universal aqueous buffer series with pH in the range of 2.4–11.8, at mercury electrode (HMDE). The evaluation of the important kinetics criteria of arylazomethine-1,2,3-triazole derivatives was performed experimentally in Britton Robinson universal buffer solutions. The electrons which participate in the electrode reaction of arylazomethine-triazole derivatives were calculated using controlled potential coulometry. Theoretical cyclic sweep voltammograms were generated via software of digital simulation for testing the accuracy of the experimental chemical and electrochemical criteria. The good comparison between the generated and experimental cyclic sweep voltammograms confirms the accuracy of the electrochemical parameters and the proposed electrode mechanism. A good agreement between the theoretical voltammograms and the experimental one of EqCirr model of the 3-((1H-1,2,3-triazol-4-ylimino)methyl)phenol derivative (1) at pH 3.2 and a scan rate of 1000 mVs-1 is shown in the following figure. The obtained theoretical and experimental chemical and electrochemical parameters confirm the accuracy of the proposed mechanism. Keywords: Arylazomethine-triazole ; Convolution transforms ; Chronoamperometry ; Digital simulation Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-019-00788-9
Chemical Papers 73 (9) 2353–2362 (2019) |
Friday, May 09, 2025 |
|||
© 2025 Chemical Papers |
||||