Validated and rapid measurement of the ferric reducing antioxidant power in plasma samplesMaria L. Gonzalez-Rivera, Flavio Martinez-Morales, Angel J. Alonso-Castro, Juan F. Lopez-Rodriguez, Juan R. Zapata-Morales, Saray Aranda Romo, and Othoniel H. Aragon-Martinez Autonomous University of San Luis Potosi, San Luis Potosi, Mexico
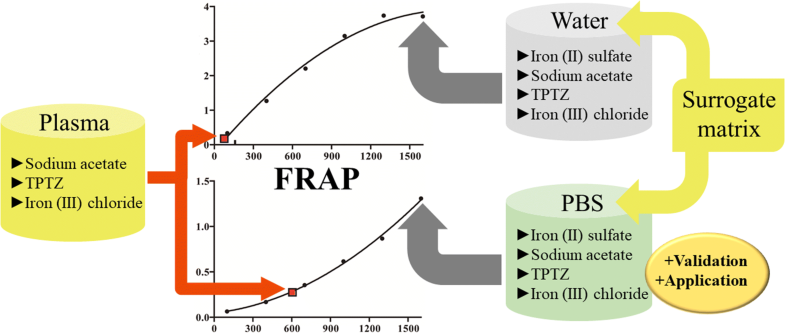
E-mail: hugo.aragon@uaslp.mx Abstract: The ferric reducing antioxidant power assay was developed using rat plasma samples and validated according to the Food and Drug Administration guidelines as well as strategies for the lack of endogenous compounds-free samples of matrix. For validation procedures, the phosphate buffered saline solution was used as the artificial matrix because this led to a direct interpolation in calibration curves. The absorbance responses and antioxidant activities in curves showed a second order polynomial relationship (R2 value = 0.9982). The precision, accuracy, and stability of the method ranged from 1.7 to 7.2%, 89.8 to 100.0%, and 82.7 to 111.6%, respectively. This assay had a short time of analysis (96 samples per min) and absence of interferences during the spectrophotometric monitoring. For the application of the method, the plasma antioxidant capacity, blood distribution of levofloxacin, and biometry hematic were evaluated in samples obtained from rats under different experimental conditions. The in vitro condition applied to blood samples increased the plasma antioxidant capacity and volume of erythrocytes, whereas diminished the levofloxacin concentration in these cells. The high antioxidant activity was produced by a high amount of inosine, which in turn was caused by high oxidative stress leading to an impaired blood distribution of levofloxacin and erythrocyte swelling. This assay is a validated and rapid biomarker for the evaluation of the total antioxidant capacity in plasma samples. Keywords: Ferric reducing antioxidant power ; Bioanalytical validation ; Surrogate matrix ; Erythrocyte dysfunction ; Fluoroquinolone Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0512-9
Chemical Papers 72 (10) 2561–2574 (2018) |
Thursday, April 03, 2025 |
|||
© 2025 Chemical Papers |
||||