(E)-N-(pyren-1-ylmethylene)benzenamine: efficient promoter for additive-free palladium catalyzed aerobic oxidative coupling of arylboronic acids and terminal alkynesJagadeesan Lakshmipraba, Rupesh Narayana Prabhu, and Victor Violet Dhayabaran Post Graduate and Research Department of Chemistry, Bishop Heber College, Tiruchirappalli 620 017, India
E-mail: lakshmiprabachem@gmail.com Received: 6 December 2019 Accepted: 2 April 2020 Abstract: AbstractA highly productive protocol for the synthesis of internal alkynes by the carbon–carbon cross-coupling reactions of electronically different arylboronic acids with substituted phenylacetylenes was described by employing (E)-N-(pyren-1-ylmethylene)benzenamine with Pd(OAc)2. The influence of reaction parameters such as solvent, base and reaction temperature in this carbon–carbon cross-coupling reaction was also investigated. The substrate scope could be expanded to electron-poor alkynes, for which the conventional Sonogashira reaction gives poor yields. Moderate to excellent yield was obtained in the oxidative Sonogashira-type coupling reaction. Graphic abstract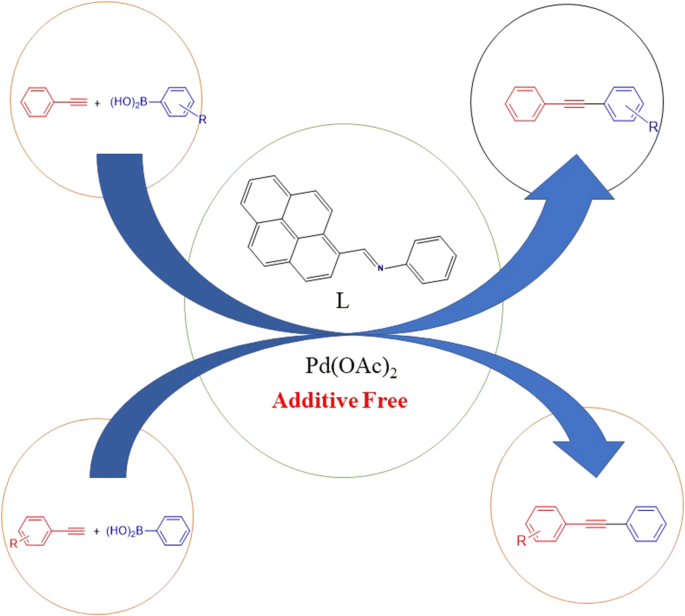 Keywords: Schiff base; Palladium-catalyzed; Oxidative Sonogashira-type; C–C coupling; Diaryl acetylenes Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-020-01156-8
Chemical Papers 74 (10) 3661–3669 (2020) |
Tuesday, November 26, 2024 |
|||
© 2024 Chemical Papers |
||||





